Background
Introduction
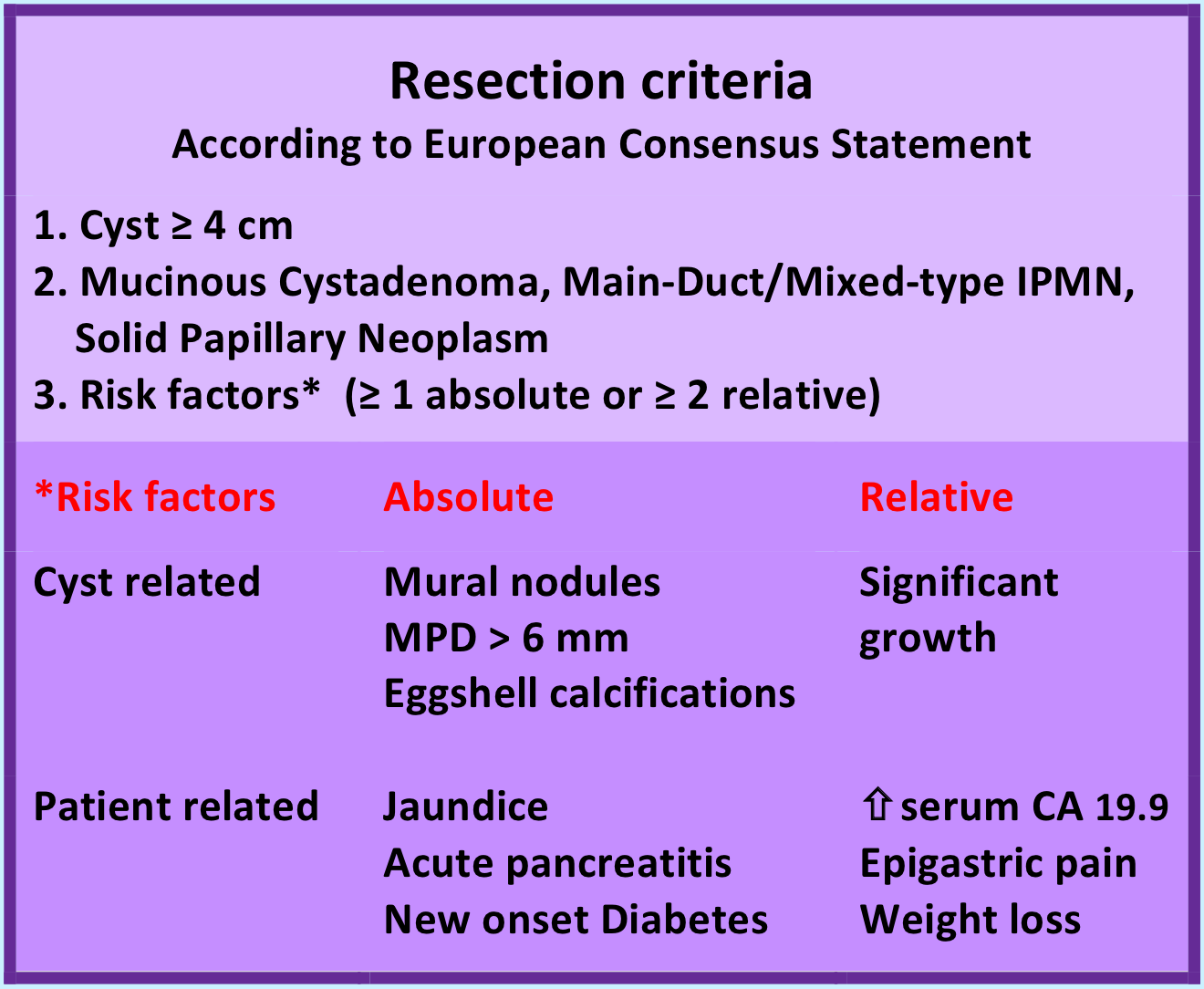
An asymptomatic pancreatic cyst is a common finding in this time of elaborate imaging. Although the malignant potential of these cysts is expected to be small, an intensive surveillance strategy is propagated. In 2013 and 2018, a group of European experts formulated a consensus statement, recommending lifelong follow-up with imaging (preferably MRI) and the determination of serum CA 19.9. Although scientific evidence is lacking, these recommendations are amply followed worldwide.
Objectives
The PACYFIC study is conducted to establish the yield of the current pancreatic cyst surveillance and to identify possible alternative, more (cost-)effective surveillance strategies.
The study is designed as a prospective international observational cohort study that will run for a period of 15 years.
Primary; the number of patients that will:
1. Reach an indication for cyst resection
2. Are diagnosed with a malignant cyst (high-grade dysplasia or carcinoma)
Secondary; to determine:
3. The outcome of patients with an indication for cyst resection
4. Cyst evolution
5. The perceived burden of surveillance of participants
6. Possible risk factors for malignancy
7. Predictive biomarkers for malignancy
8. The optimal surveillance strategy
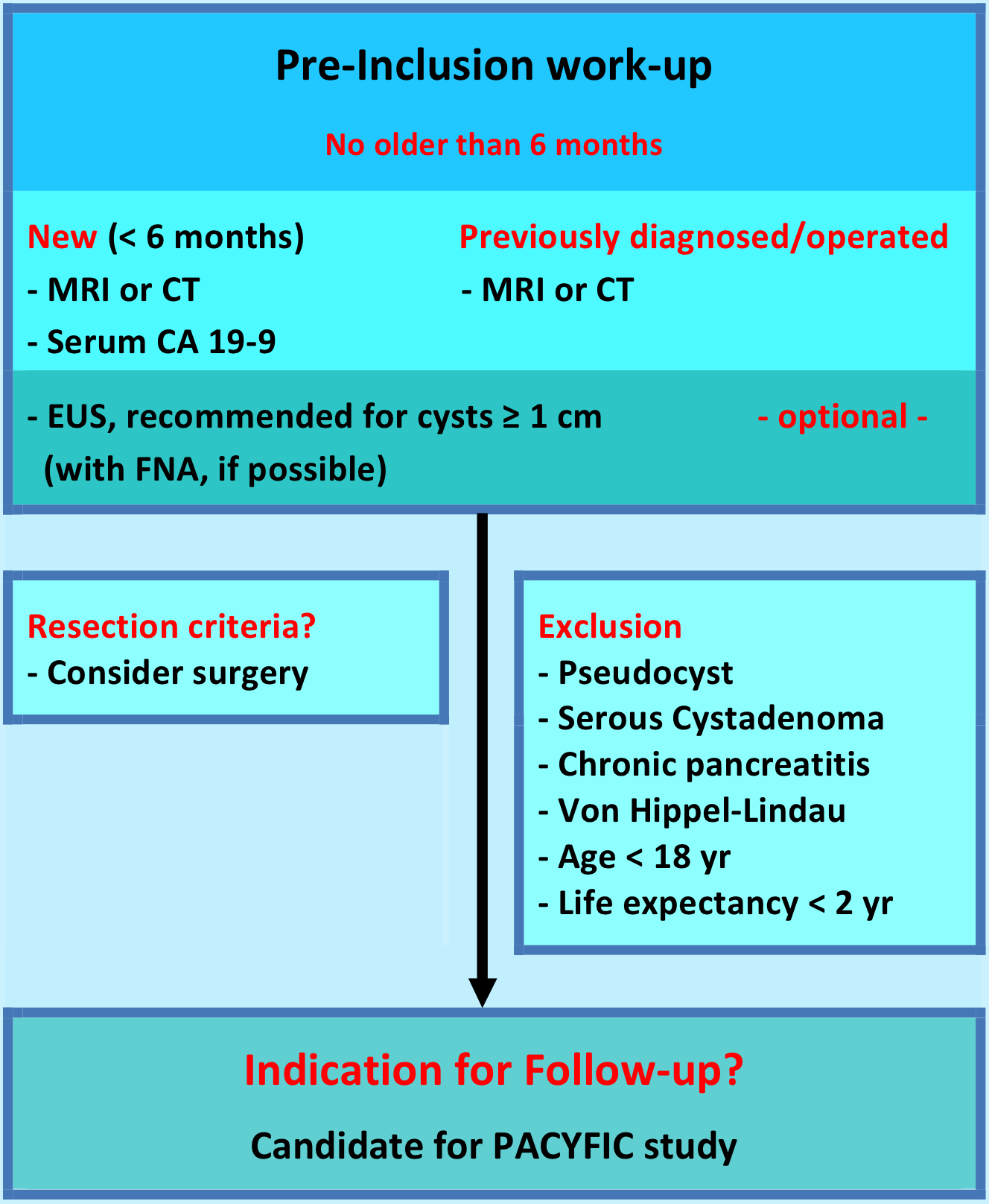
Patients & pre-inclusion work-up
Inclusion criteria
Any patient requiring pancreatic cyst follow-up;
1. New cyst (diagnosed < 6 months ago)
2. Previously diagnosed/followed cyst
3. Operated IPMN
Cyst surveillance & study procedures
Cyst surveillance
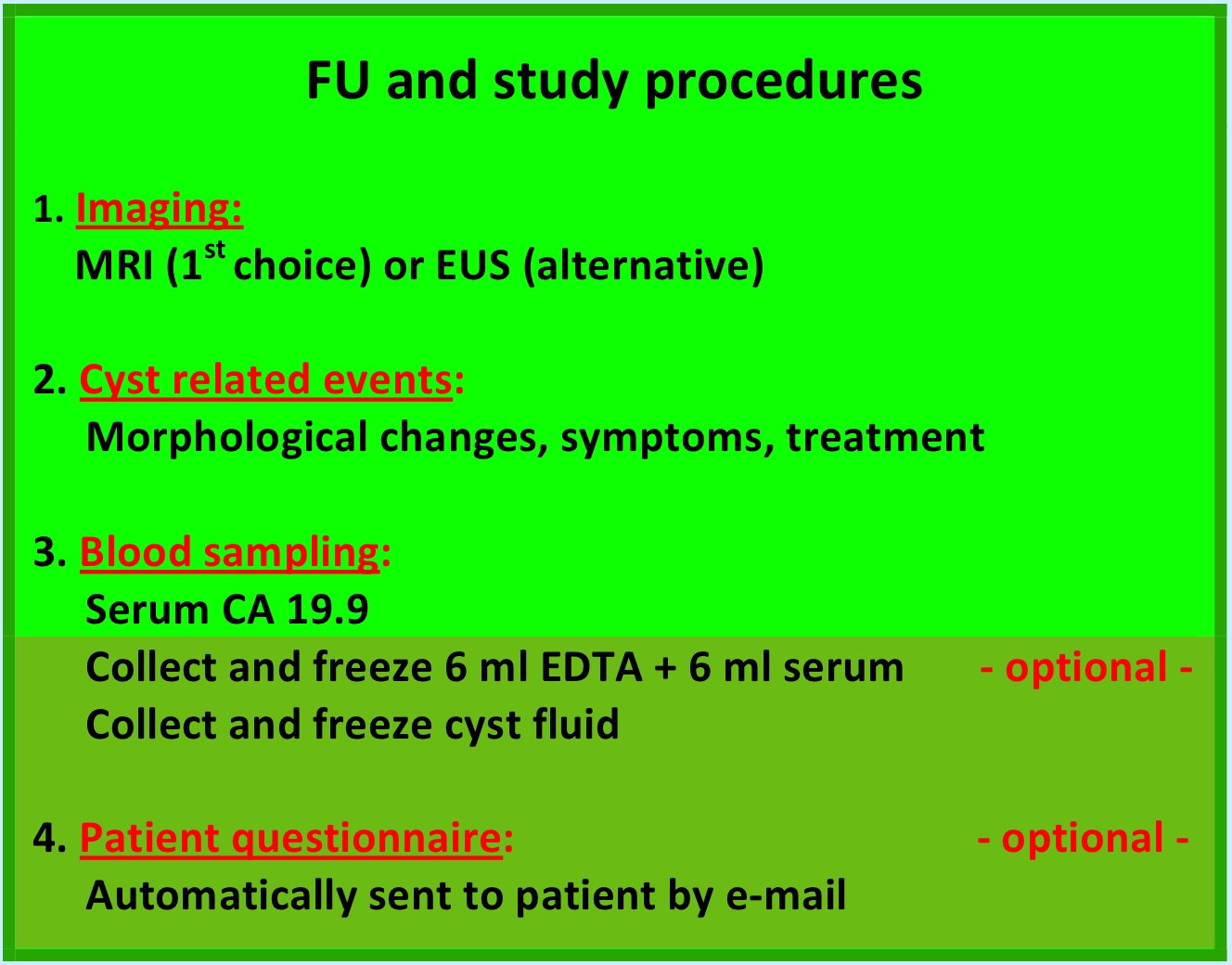
Cyst surveillance remains in the hands of the treating physician and will be carried out at the hospital of origin. The European experts consensus statement recommends yearly follow-up by MRCP (1e choice) or EUS (alternative) and serum CA 19.9. In the first year, 6-monthly check-up is recommended for cysts ≥ 1 cm in size.
Study procedures
Collection and storage of Biomaterial
Biomarker research is a promising part of the PACYFIC study, therefore, we aim to collect and freeze both serum and cyst fluid. The study team will provide in the transportation and processing of these samples. This part of the study is optional. You can also participate in the PACYFIC study, without contributing to the collection of biomaterial.
Blood samples
Each time blood is drawn for CA 19.9 determination, two additional 6 ml vials need to be collected; an EDTA and a serum vial. For this, an order form can be downloaded from our website (Blood collection form). These vials must be labeled with the provided PACYFIC stickers, filled out with either a study number, or a patient number (both is not permitted for privacy reasons). The EDTA sample needs to be frozen at -20 degrees Celsius. The serum sample requires centrifugation first, and subsequent freezing at -80 degrees Celsius.
Cyst fluid
All cyst fluid that remains after standard diagnostic work-up should be stored at- 80 degrees Celsius. Both pure cyst fluid and supernatant (remainder fluid after centrifugation for cytology) are suitable for storage. These vials must also be labeled with the PACYFIC stickers and patient or study number.
Patient questionnaires
Only patients with a newly diagnosed cyst will be asked to fill out questionnaires regarding the burden of surveillance. This part of the study is optional. A patient can also participate in the study, without joining the questionnaire. We will provide your patient with a link to the online questionnaire by email. A baseline questionnaire will be sent, just before the first FU visit, concerning their medical history and expectations of surveillance. Thereafter, patients will receive a standard questionnaire before the second FU visit, and after each FU visit during the next 3 years.
Downloads
-
- Patient Questionnaire Inclusion (doc)
-
- Patient Questionnaire FU (doc)
-
- Biomaterial Protocol (doc)
Resection criteria, as proposed by the European Experts Consensus Statement
To view the European consensus statement, please click here.